Burning fossil fuels causes a considerable amount of greenhouse gases to be released into the atmosphere. Therefore, the world is shifting toward green energy because it emits fewer greenhouse gases. As a result, a variety of renewable energy sources including wind energy, solar energy and hydropower are being explored as alternative energy supplies.
The five categories of power storage technologies that can be used for a wide range of applications are mechanical, electrical, thermal, battery and chemical. Due to a variety of attractive features, the battery energy storage method is one of the most preferred forms of energy storage.
Battery Energy Storage
The batteries are constructed of stacked cells that convert chemical energy to electrical energy & vice versa. The energy capacity of the majority of battery types are fixed during the battery design. The battery technology is now undergoing major progress. Different battery types are being created, some of which are currently on the market and others of which are still in the prototype phase.
Working of Battery Energy
Anode and cathode are the two electrodes that normally make up a battery. The positive end of the battery is made from cathode, while the negative end is made from anode. Anions typically travel from the cathode to the anode when the battery is connected to an electrical source. The battery is being charged in this scenario. The cation switches from moving toward the anode to the cathode during the discharge phase of the battery as shown in the figure. 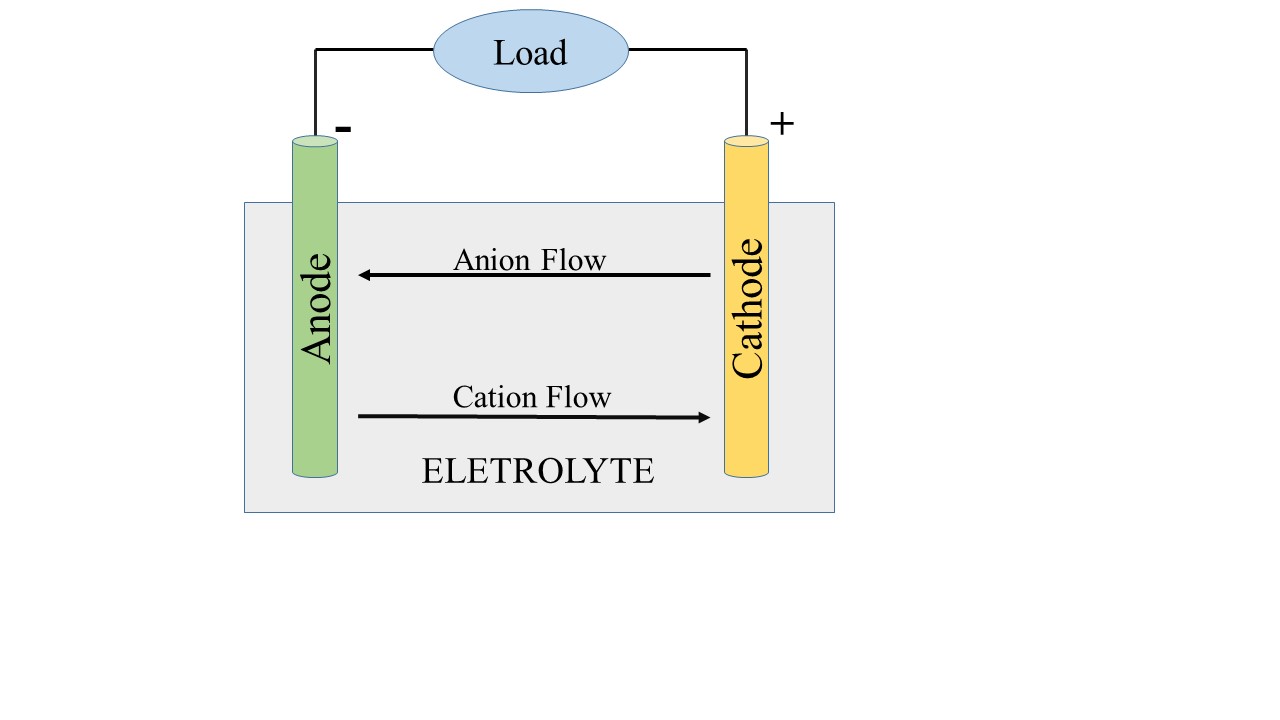
Types of Battery Energy Storage
The following are different categories of battery energy storage devices:
- Lead-acid battery
- Nickel- cadmium battery
- Nickel-metal hydride battery
- Sodium-sulfur battery
- Lithium-ion battery
1. Lead-acid battery
In 1859, French physicist Gaston Plante created the lead-acid battery. The lead-acid battery energy storage method is the most developed and least expensive of all battery power storage methods was invented by French physicist Gaston Plante in 1859. For many years, lead-acid batteries have been used as battery backup in power plants and transformer sub – stations. But when there is a deep or quick discharge, though, its performance is greatly reduced.
Reduced energy density, minimum power density, prolonged charge times, short lifetimes, and high self-discharge rates are all problems for lead-acid batteries. Additionally, despite their low cost, these batteries have not been widely used in the energy storage industry all over the world in past few years. This is because they are likely to pollute the environment.
The following reaction illustrates the reversible electrochemistry reactions in a lead-acid battery.
Pb + PbO2+2H2SO4 ⇌ 2PbSO4+2H2O
2. Nickel- cadmium battery
In 1899, Waldemar Jungner created the nickel-cadmium battery. It has several advantages over lead-acid batteries, including a longer lifespan, improved low-temperature performance, and higher charge/discharge rates. The nickel-cadmium battery traditionally dominated the market for backup and portable power sources due to these remarkable properties. The sealed and the vented are two main types of nickel-cadmium batteries.
Typically, sealed nickel-cadmium batteries are utilized in remote controls, lighting, and other electronic equipment. These batteries never emit any gases, unless a problem arises. The same operating principles apply to vented nickel-cadmium batteries except gas is released during overcharging or rapid discharge.
The following reaction illustrates the reversible electrochemistry reactions in a nickel-cadmium battery.
2NiO(OH)+Cd+2H2O ⇌ 2Ni (OH)2+Cd (OH)2
3. Nickel-metal hydride battery
First time Nickel-metal hydride batteries were manufactured in 1986 and became widely commercialized in 1989. Its design resembles that of a nickel-cadmium battery quite a bit. But they were less expensive and more environmentally friendly because metal Cd replacement is feasible.
Nickel-metal hydride batteries also have a number of additional huge benefits over nickel-cadmium batteries, including little memory effect, long lifespan, outstanding performance across a wider range of operating temperatures, maximum charge rates, & higher energy densities. Their energy density is around 50% greater compared to nickel-cadmium batteries.
The following reaction illustrates the reversible electrochemistry reactions in a Nickel-metal hydride battery.
Ni(OH) +MH⇌Ni(OH)2+M
4. Sodium-sulfur battery
A particular kind of battery made from molten metal and sodium and sulphur is known as a sodium-sulfur battery. This kind of battery is made of low-cost materials and has a high power density, good charge/discharge efficiency (70-90%), and extended cycle life.
However, due to their operational temperature range of 300-400°C and the highly corrosive properties of sodium polysulfide discharge products, such cells are particularly appropriate for large-scale and industrial applications.
The following reaction illustrates the reversible electrochemistry reactions in a sodium-sulfur battery
2Na + 4S⇌ Na2S4
5. Lithium-ion battery
Due to their significant potential for offering effective energy storage & sustainable development, lithium-ion batteries have become increasingly popular. Nowadays, lithium-ion batteries are employed in both electric and hybrid automobiles as well as portable gadgets like cell phones and laptops. In reality, lithium-ion batteries are becoming more and more well-liked for all of those applications because to their great performance and energy density.
Additionally, lithium-ion batteries presently lead the market for batteries used in portable electronics due to their specific features above other battery systems, including their high specific capacity & voltage, excellent cycling performance, low self-discharge, & large operating temperature variety. Yet, their poor safety performance presently poses a substantial barrier to the continued development of the lithium-ion battery market and its major consequences for EVs.
The following reaction illustrates the reversible electrochemistry reactions in a lithium-ion battery.
LiCoO2+C⇌Li1-xCoO2+LixC
Application of Battery Energy Storage
Battery energy storage is crucial to the energy shift from non-renewable to green energy. We can provide peaceful, emission-free energy in places with poor grid connectivity or for customers who want to operate as sustainably as possible by storing renewable energy in batteries.
-
Peak Shaving
It is the process of altering the energy supply structure, so that energy throughout the peak times is shifted to off-peak times. In other words, when there is a surplus of renewable power, the power is stored in batteries & supplied to the system when there is a high demand.
-
Emergency Back-up Power Generation
Battery energy storage systems can serve as emergency power sources in the event of a network outage to provide sufficient energy until the system is restored.
-
Uninterruptible Power Supply (UPS)
The battery storage system is required to supply power to the system in the event of a power outage or overload. UPS systems respond quickly by supplying power for a few minutes to several hours. They are intended to rapidly and instantly deliver backup power in the event of a power supply outage or other unfavorable circumstance.
-
Integration with Renewable Energies
Renewable energy sources are intermittent, they may be available when they are not needed and may not be available when they are needed. Regrettably, energy providers are unable to forecast or regulate the maximum power from such sources with any degree of accuracy. As a result, battery storage would help the most sustainable energy facilities, such as wind turbines & solar cells.
Cite this Article
| Author: | Scholars Harbor |
| Year: | 2022 |
| Title | What is Battery Energy Storage ?- Working, Types And its Applications Explained |
| Publisher: | Scholars Harbor |
| URL: | https://scholarsharbor.com/what-is-battery-energy-storage-working-types-and-its-application-explained/ |













Discussion about this post